
A biopharmaceutical company specializing in the development of gene therapies had an impressive late-stage clinical program progressing several drug candidates towards commercial approval. However, the existing quality system would need to be revamped to handle the potential marketing of multiple approved biologic and drug products.
The company needed to help develop a more robust quality system to fit the commercial needs of this growing organization. It also needed a resource partner that could develop project plans, supply temporary experienced, top-level consultants to address the additional workload and manage the resources and deliverables to ensure the development and implementation of the new quality management system.
Challenges
The additional demands of commercial expansion taxed the bandwidth and expertise of the company’s quality system, quality assurance and quality control teams.
Moreover, the expansion of the quality system would require significant investment in time and resources including process mapping and development, documentation creation, systems integration and improvements, training and other critical activities. The company would also need time to find, recruit and onboard permanent employees for newly identified positions.
How We Helped
The client and Black Diamond Networks collaborated on a comprehensive strategy to identify the compliance gaps and associated risks, understand the needs of the organization and build out the quality system. Black Diamond developed the project plans, identified and addressed the resource gaps, managed the resources and milestones, and ensured the execution of the project.
We also sourced contract-to-hire and consulting personnel to bridge the gap until permanent hires could be transitioned in the new roles created by the changes to the quality system. In total, we supplied over 20
professional resources with the experience, expertise and drive to ensure the success of the project.
Successful Results
Black Diamond Networks aided in the design, development and implementation of an improved quality system covering preclinical, clinical and commercial operations. This successful project created a lasting
impact through improved processes, more compliant procedures, stronger documentation and better trained and prepared personnel.
Working with Black Diamond
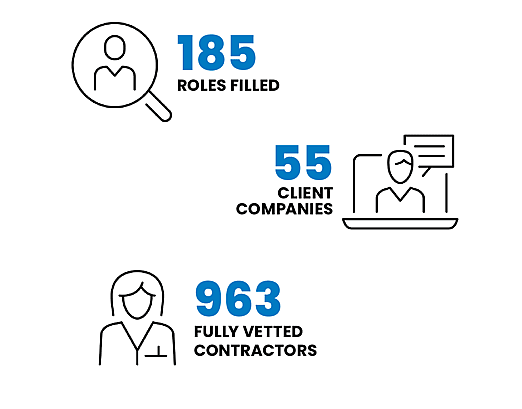 In the last year, Black Diamond Networks filled 185 roles at almost 60 cell and gene therapy or tissue engineering companies.
In the last year, Black Diamond Networks filled 185 roles at almost 60 cell and gene therapy or tissue engineering companies.
How do we do it?
Our staffing model is wired for speed and precision, thanks to a cultivated community of experts in life sciences, engineering, and technology.
In fact, we have almost 1000 contractors with cell and gene therapy and tissue engineering experience in our network.
These highly-skilled candidates are already fully vetted and Black Diamond approved.
Typical roles we fill for our cell and gene therapy clients
- Clinical Development Scientists
- CMC Experts
- Regulatory Professionals
- Analytical Development Scientists
- Process Engineers
- Manufacturing Engineers
- Project Managers
- QA/QC Specialists
- MS&T/MSAT Specialists
Black Diamond Networks actively supports our clients across the cell and gene therapy sector by providing the hard to find talent needed throughout the entire process, from design and development to commercialization.
Clients in the life sciences industry turn to us, time and time again, thanks to the quality of our candidates and the speed we can fill their most specialized roles.
We understand that each company and project is unique, which is why we have a flexible staffing model, from traditional staff augmentation to complete project solutions, so you get the support you need, where you need it.
Need project support?
We’re supporting clients on a variety of scales – by overseeing and staffing multi-site projects to sourcing individual subject matter experts. How can we help you? Let’s discuss.

