June is Alzheimer’s and Brain Awareness Month. With many various ‘awareness’ months competing for our scarce attention, why is this one so important?
By the numbers:
Number of drug approvals for Alzheimer’s Disease in the last two decades: 2.
Memantine (Namenda) in 2003
Aducanumab (Aduhelm) in 2021
That’s an average of about one every decade. And zero prior to that.
Number of cases of Alzheimer’s:
6,000,000 (US)
50,000,000 (global)
As the population ages, that window will continue to expand and grow. It accounts for 60%-80% of dementia cases, and is the 6th leading cause of death in the US.
This is a critical area of research and treatment. We all, with certainty, have known or will know someone suffering from Alzheimer’s Disease.
60 Seconds of Science on Alzheimer’s
What do we know about Alzheimer’s disease? What causes it?
It used to be thought that dementia in old-age was the natural progression of the mind. As more people began living longer through the 1800s, features began to appear which are synonymous with the condition we now call Alzheimer’s Disease. It was categorized in 1906 by Dr. Alois Alzheimer, who first presented on a patient case at a conference of German psychiatrists. There he described the “neurofibrillary tangles” and “senile plaques” that are considered to be the hallmarks of the disease. For his work on recording patients and their decline with the condition, it was named for him (in 1910).
In more recent years, we have come to learn that Alzheimer’s Disease refers to a spectrum of disease (which can be more-rapidly progressing or less-rapidly progressing) and seems to be associated with a particular set of molecular causes. But reflect on this for a moment: We’ve known about plaques in brain tissue for 115 years. And have but two drugs approved to contend with it.
The Role of Beta Amyloid
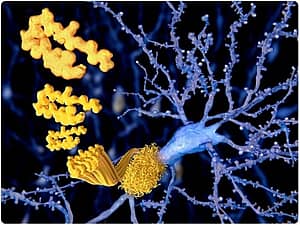
There is a protein-based molecule called β-amyloid (pronounced “beta amyloid”), which forms plaques in the brains of patients with Alzheimer’s. For this reason, drug therapies to try to reverse the course or cure the disease have focused on this so-called ‘amyloid hypothesis.’ The trouble has been that there have been an incredible number of drug therapies which didn’t survive clinical trials whose mechanism of action was to directly target and attack these beta amyloid plaques.
Many drugs in Phase 1, 2, and also Phase 3 have ended up in the pharmacologic graveyard of ineffective therapies for Alzheimer’s (and specifically, β-amyloid), and have accrued costs in the tens of billions of dollars for the sponsor companies.
So pharmaceutical companies are rightly cautious now about trying to go about targeting amyloid plaques as a treatment for Alzheimer’s Disease.
Current Events: Enter Aducanumab, and a Polarized Debate in the Industry
About a decade-and-a-half ago (and from an interesting contract signed with Neurimmune in 2007), Biogen was working diligently on the amyloid hypothesis. Also in their targets were genetic markers associated with Alzheimer’s Disease (apolipoprotein E, or APOE ε4) which was thought to have a genetic association with the instigation of late-onset Alzheimer’s. The drug candidate they were investigating, aducanumab, is a monoclonal antibody that targets a particular surface feature on amyloid beta molecules. Early trials seemed to show a good targeting effect of amyloid plaque molecules by the drug candidate, and the hope would be that aggressive targeting of beta amyloid would reliably lead to a reduction in clinical symptoms of Alzheimer’s in patients. This type of attempt is called a ‘surrogate endpoint’ – one which is reasonably likely to predict a clinical benefit.
In March 2019, Biogen was working on two Phase 3 trials in order to then apply for aducanumab’s approval with the FDA. However, both trials were stopped halfway through because they hit prespecified ‘futility’ criteria (agreed-upon endpoints of non-effectiveness that if met, would stop the trial).
The issue has been that, while the Biogen team had been scrambling to look at subgroups and sub-stratifications of the patients who have been treated with the active drug to try to identify any reliable efficacy signal from the trials, Wall Street investors and the FDA’s own advisory panel had been suggesting that Biogen is trying to squint very hard to detect an effectiveness signal which simply isn’t there.
Biogen analyzed the results of the trials and suggested that the high-dose patient population in the second trial had in fact experienced a statistically significant clinical benefit when measured against the placebo (a 22 percent reduction of clinical decline as measured on a chosen clinical scale). However, it was a benefit absent from the high-dose population in the first trial as well as the low-dose population in either trial. So, perhaps a statistical fluke.
While some at the FDA (and especially at their advisory committee) didn’t think there was good enough efficacy data, Dr. Al Sandrock, Biogen’s EVP of R&D said, “I believe the drug works.” Though it is sometimes hard to separate bias when you’re a corporate officer or employee in situations like this (and why SEC rules exist in the first place), Dr. Sandrock was also a seasoned physician and neurologist – so he’s seen some of these patients personally through the good and the bad.
This has also set off a somewhat political and very polarized debate about the nature of FDA’s oversight in the drug development process, how much of the decisions are made by the weight of science versus the weight of illusory factors or conflicted interests.
Fast-forward to June 2021, and the FDA has granted approval to Biogen for aducanumab (brand name Aduhelm). To-date, three members of this advisory committee to the FDA have resigned their posts as a result of the approval decision for Aduhelm being based on partisanship and not science. But what is clear is that the lack of efficacy for Aduhelm isn’t so clear; Biogen did pull together some compelling clinical data suggesting benefit for certain patients. However, on an 18-point scale measuring subjectively the progress of the patients in their disease trajectory, Aduhelm seems to only have an effect size of a fraction of a point (on the 18-point scale). So while this result may hold up as statistically significant (and therefore, really ‘there’), it may continue to be not an overwhelming improvement for the patients’ clinical trajectory.
The other issue that has raised an ethical specter is that Biogen has priced Aduhelm at $56,000 per year. This is about 6x more than the drug was initially valued at. There are a couple reasons why this is a problem: First, is that many people wouldn’t be able to afford the therapy. And second, the Center for Medicare and Medicaid Services (CMS) will have a number of challenges coming up as far as deciding who is eligible – or ineligible – for the treatment. This is especially complicated by the fact that the FDA gave a broad approval for its use (putting no restrictions on how this is diagnosed, such as by PET), which makes declining it for certain patient populations more difficult. This could make all of the estimated 6 million Americans with Alzheimer’s suddenly eligible, and many more who could be inadequately diagnosed. Some financial scenarios are modeling that this may place a tremendous burden on Medicare funds (especially Parts B and D), if they are reimbursing for all of the requests (spurious and otherwise) for the treatment.
Reasons for Hope
Still, I think the most important voice in the debate over treatment of Alzheimer’s in general – and Biogen’s recent approval in particular – has been from the President and CEO of the Alzheimer’s Association, Harry Johns, who just recently said, “This approval [of Aduhelm] is a victory for people living with Alzheimer’s and their families.” He continued to say,
“This is the first FDA-approved drug that delays decline due to Alzheimer’s disease. This means individuals may have more time to actively participate in daily life, have sustained independence and hold on to memories longer. We can experience longer — the relationships we hold most dear — our families and friends.”
So while there will continue to be vociferous debates about the nature of the approval, and the weight of the scientific evidence vis-à-vis the clinical trials, he’s exactly right: oftentimes in medicine, we don’t go from ‘Zero’ to ‘Cure’ in one treatment, but instead it is an evolution of therapies which leads to a growing body of evidence and increased understanding of the disease itself. As we treat more patients, we help them and we learn more in the process to improve the future.
In supporting the very infrastructure and fabric of the industry’s scientific pursuits, we are helping to bring these and other remarkable therapies to the patients who need them.
We’ve got to start somewhere. The present is the past from the perspective of the future. Let’s create a better future, since we’ll all be there soon.


