
President Biden has recently nominated Robert Califf to be the Commissioner of The US Food and Drug Administration. This will bring with it substantial changes in 2022 (and 2023) not only within the FDA’s internal functioning, but broadly across the pharma and medtech industries.
Organizational Changes Bring…Change
There is a relatively obscure conjecture in business operations called “Conway’s Law.” It suggests that when organizations construct communication hierarchies, these hierarchies resemble smaller versions of the larger organization itself.
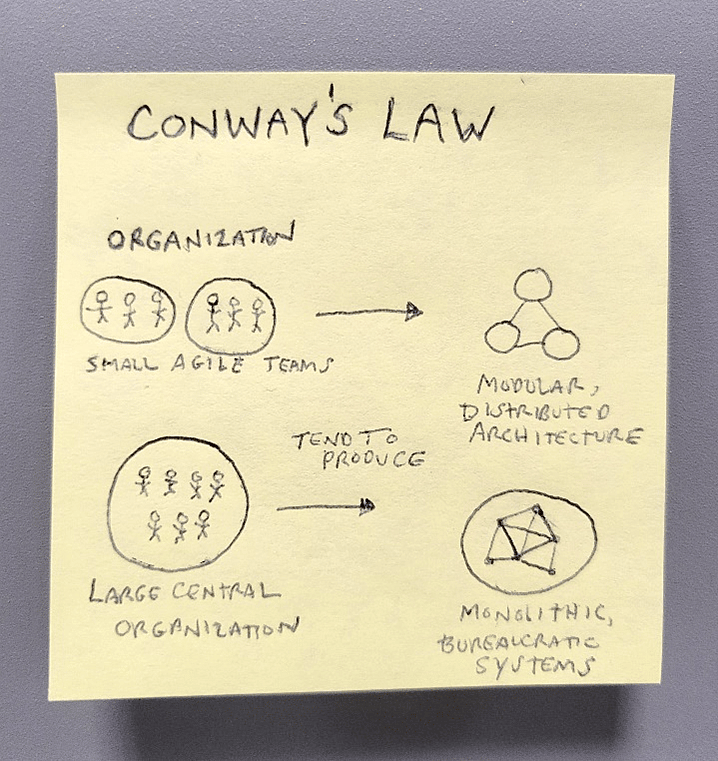
In essence, like Russian Nesting Dolls (matryoshka), each layer down of an organization makes mini organizations that resemble and communicate like the layer above.

The interesting connection here is that when companies change their leadership, this often catalyzes wholesale changes to these structures. These moments often bring brand new change throughout the organization, as well as work on items that had been left undone. This happens with varying degrees of shocking predictability.
So what will Robert Califf’s appointment change at the FDA? And why does it matter? For two major reasons:
- Leadership will change the organization
- What the FDA does – and finds important – will make the industry fall in line to follow suit
The Leadership Factor
Changing leadership with respect to time leads to a non-zero outcome that can be either positive or negative. But in either case, the organizations amid leadership changes don’t remain static.
We often can’t know the outcome unless we look in retrospect after the new leadership experiment has had a chance to run its course. So whether Robert Califf’s appointment will be a success or otherwise has a lot to do with providence, chance-y situations, and many other things outside of his control (not least of which are lobbyists, budgetary constraints, activists, and so on). But we can focus on element #2 from our list above: planning for industry’s change in the forecasted direction(s) of the FDA’s changes.
Small Moves Make an Overall Strategy
The Regulatory Chessboard
When shifts occur within the FDA (and other global agencies as well, including MHRA, EMA, with ICH documents, etc.), they reverberate through the industry and catalyze changes in how work is done. This creates potentially profound impacts on the chessboard of regulatory approvals.

Probably the most voluminous changes in the industry seen in some time due to a regulatory change were those associated with updates in ICH E6 revision 2, as well as changes from MDD to EU MDR (Medical Device Regulation). Literal years of change went on within the pharma and medtech industries to prepare for those updated documents and their associated new requirements. Changes in the FDA will come in-part from Califf’s interests and the directions toward which he finds it important for the agency to go. And where these directions point, so too will the industry necessarily follow.
Forward-Looking Themes At FDA
So what are these likely changes, then? Well, if I deconstruct what I know of Robert Califf and his messaging internally and externally, they begin with ‘data,’ and end with ‘data.’
First, changes to ‘how’ we measure data in the industry. This will build on existing work at FDA on the Technology Modernization Action Plan (TMAP) and Data Modernization Action Plan (DMAP), among other initiatives.
The Data Modernization Action Plan (DMAP) proposes a framework and actionable recommendations for FDA’s Data Strategy. It consists of 3 key components:
(1) Identify and execute high value driver projects for individual centers and the Agency;
(2) Develop consistent and repeatable data practices across the Agency; and,
(3) Create and sustain a strong talent network combining internal strengths with key external partnerships.
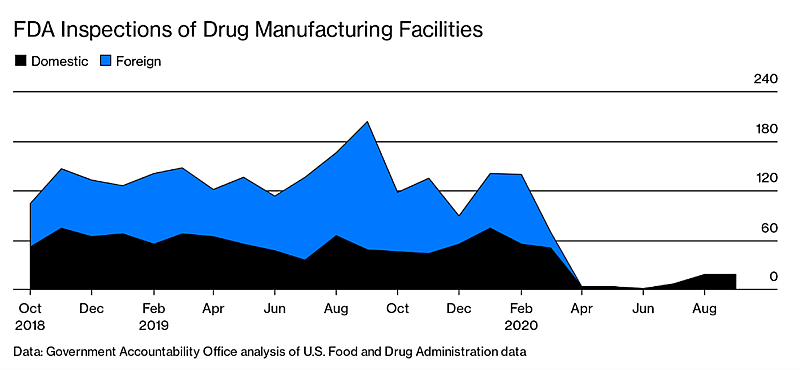
These changes will also directly and indirectly help the FDA to address the backlog of thousands of facility inspections that they are required to perform. The COVID-19 impact on domestic site inspections led to 15,000 postponed inspections for food, drug, and medical device companies. The architecture of GMP inspections from 2011 through 2020 looks like this:
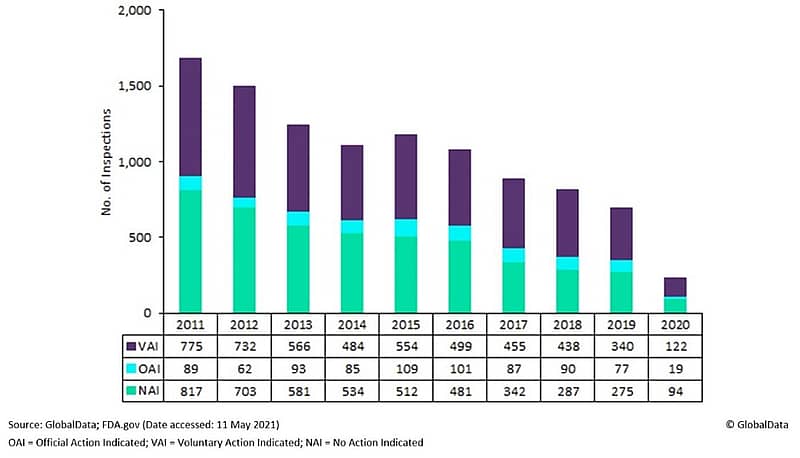
Out of 3,229 overdue backlogged inspections for pharma and medical devices, the FDA has completed over 1,000 of those which are outstanding. But these remaining backlogged facility inspections will need to take place along with the normal annual requirements to review new drugs and devices.
Planning For the Future
Past performance is a fairly mediocre predictor of future behavior. But it’s a predictor nonetheless. So if we look to some of the things that Califf spearheaded in his last run leading the FDA (as its 22nd Commissioner in 2016-2017), we can get a sense of the directionality of the future,. To that end, he had launched IMEDS (Innovation in Medical Evidence and Surveillance) in 2017, which allowed drug companies to mine patient safety data to identify potential problems with their drugs. IMEDS lists the following benefits on their website:
- Access to large volume of patient data
- Reliability of an established network
- Direct experience and expertise leveraging FDA’s Sentinel Common Data Model and tools
- Accessing and contributing to collective knowledge building around real-world evidence generation
In 2016, Califf wrote an article, with his colleague NIH Director Francis Collins, wherein he laid out a plan to create a “learning health system,” one that “that takes full advantage of digital data to help us make informed choices.”
So what I do know of his past performance and interests is that he is deeply interested in the philosophy of transforming how our industry collects and uses data. This will, as I’ve explained above, directly impact the collection and use of data in the industry in the coming years.
Califf also has been interested in toughening stances on tobacco as well as opioids, including having opioid manufacturers present different types of data showing risk-to-benefit value of their therapies and longer-term safety data, in orderto look differently at the benefits and risks of this drug class.
So, while Califf’s plans for modernizing the way Sponsors and the FDA collect and use patient data were largely shelved in 2017 when he left his office, back to this area is the most evidence-based place to go for understanding what he’ll focus on principally when he hits his stride in his renewed position. These changes to inspecting facilities, TMAP/DMAP, and other initiatives – along with changing structure in the Agency – will be guaranteed to have direct and indirect impacts on the industry.
As is always the case: chance favors the prepared.

References and Further Reading
Regan-Udall Foundation of the FDA. (2021). https://reaganudall.org/programs/imeds
Califf, R. et al. (2016). Transforming Evidence Generation to Support Health and Health Care Decisions. The New England Journal of Medicine, 375. 2395-2400.
GlobalData Healthcare. (2021). Pandemic creates 500-site backlog for FDA, despite decade of dwindling US inspections. Pharmaceutical Technology. 26May2021. https://www.pharmaceutical-technology.com/comment/pandemic-backlog-fda-inspections/
Kay, C. (2021). Drug Regulators Turn to Zoom-Style Plant Inspections During the Pandemic . Bloomberg BusinessWeek. https://www.bloomberg.com/news/articles/2021-04-14/fda-weighs-virtual-drug-factory-inspections-to-clear-backlog

