 Pharmacovigilance (PV) is a discipline and function within the healthcare industry which is focused on monitoring the safety of pharmaceutical (drug) therapies. Its name is derived from a combination of Greek (pharmakon – meaning drug) and Latin (vigilare – meaning to keep watch) and refers to the systems and practices of assessing what sorts of adverse events are associated with a patient taking a prescription drug.
Pharmacovigilance (PV) is a discipline and function within the healthcare industry which is focused on monitoring the safety of pharmaceutical (drug) therapies. Its name is derived from a combination of Greek (pharmakon – meaning drug) and Latin (vigilare – meaning to keep watch) and refers to the systems and practices of assessing what sorts of adverse events are associated with a patient taking a prescription drug.
If you think about it, there is almost nothing that could be more important in the industry than to understand and continuously monitor the data related to the safety of drug compounds. This allows us to determine if a drug is safe during clinical trials, the results of which would allow or prohibit regulatory agencies (like the FDA) from approving such a compound.
Once a drug is approved and released for commercial use to the public (called ‘Post-Marketing’), continued surveillance occurs where the manufacturer collects adverse event data to determine how serious the events are (e.g., are they mild discomfort symptoms, stomach upset, etc…. or much more severe like anaphylaxis, which is a serious hypersensitivity reaction that could be fatal).
Why should we care?
Several drugs have been withdrawn from the market as a result of safety data coming in after it was in wide distribution and use by the public. At the center of those analyses were the PV experts monitoring and analyzing incoming data to try to figure out where problems actually existed.
Here’s an example: the pain reliever drug Vioxx was withdrawn after it had been used because it was associated with an increased rate (4x) of cardiac problems (myocardial infarction), and this, among other reasons, was cause for its withdrawal:
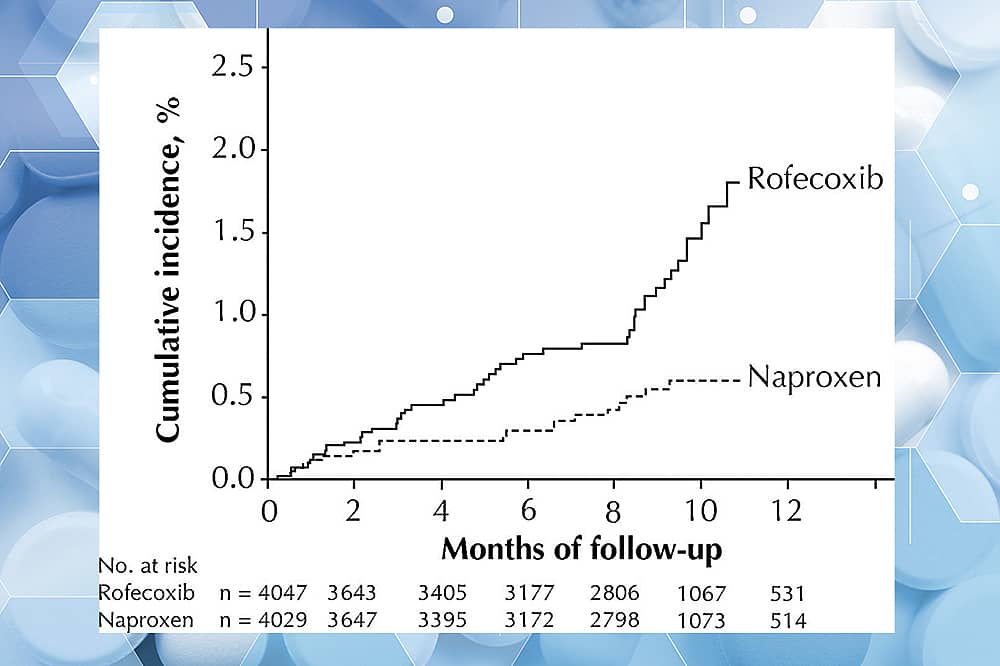
We only know these unfortunate stories of drug reactions to be true because of the pharmacovigilance data upon which they rest.
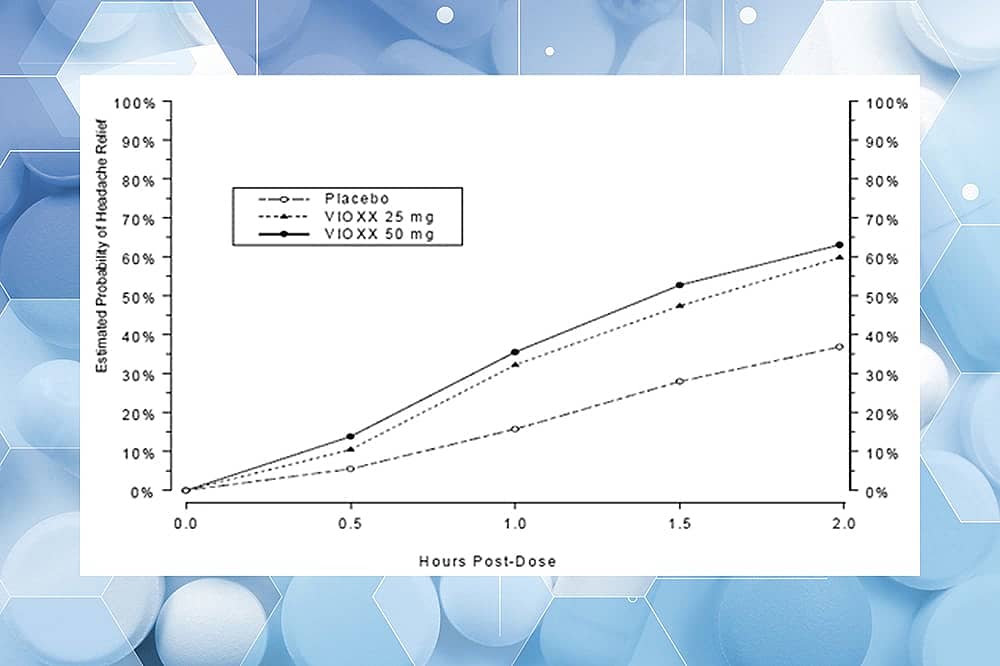
But it worked great for headache! See difference above in performance at headache relief between placebo (dashed line with circles) and Vioxx (25 mg and 50 mg, solid triangles and circles); There’s significant separation in effect after about half an hour.
Here’s another example for you: If you’ve ever had trouble sleeping, there are treatments for that (including Ambien (zolpidem), Lunesta, Intermezzo, etc.). Interestingly, and maybe unsurprisingly, it was discovered that women were potentially being overdosed with one of these drugs (zolpidem): Some studies suggested that women were at a 5x greater risk of driving impairment after taking a typical 10 mg dose. So the dose of zolpidem for women was reduced by 50% as a result of these postmarket pharmacovigilance findings.
With the review and approval of ALL drugs, there is a ratio of a benefit (to the patient, and how they feel, function, or survive) to a risk (to the patient, and what they could incur by taking the drug). Approved drugs are NEVER risk free but have a FAVORABLE profile in risk-to-benefit ratio. In the case of Vioxx, it was determined that, even though it was eminently effective for certain types of pain relief, its cardiac risk profile was too great for it to remain on shelves for uninhibited public consumption.
Pharmacovigilance in the age of COVID-19
An increase in the prevalence of chronic diseases has led to an increase in drug consumption worldwide, thereby increasing the demand for new drug development and extensive clinical trials. In fact, in 2019, the global pharmacovigilance market size was estimated at almost $5 billion (USD), and continues to grow.
In addition to this, with vaccine development suddenly in the spotlight due to the global pandemic, pharmacovigilance will be under even more scrutiny as regulations change and patient safety is put front and center. I’ll be delving into this and more at Virtue Insight’s Pharmacovigilance conference, representing Black Diamond Networks in discussions on the following:
An overview of Regulatory expectations for Pharmacovigilance
- Impact of the pandemic on PV
- Key changes to regulations regarding PV as a discipline
- PV Systems legislation – updates on current and future states
Patient Safety in Pharma
- Approaches for Patient Centricity in PV (putting the patient at the top of the list)
- Effects on patient safety in PV as a result of COVID-19
- New technology and other future approaches to PV practices
We’re keeping an eye on the cutting-edge of this discipline and more across life sciences. Why? So BDN clients are getting industry-leading support and consultation that can adapt to the quickly changing world where we live and work every day.

